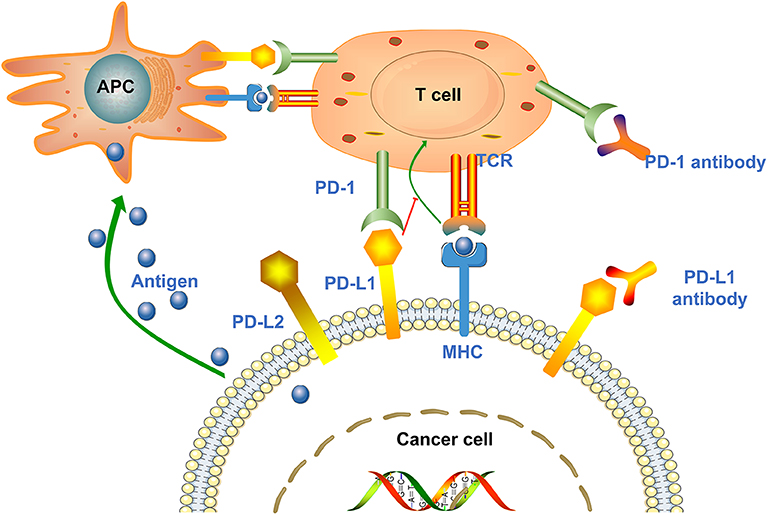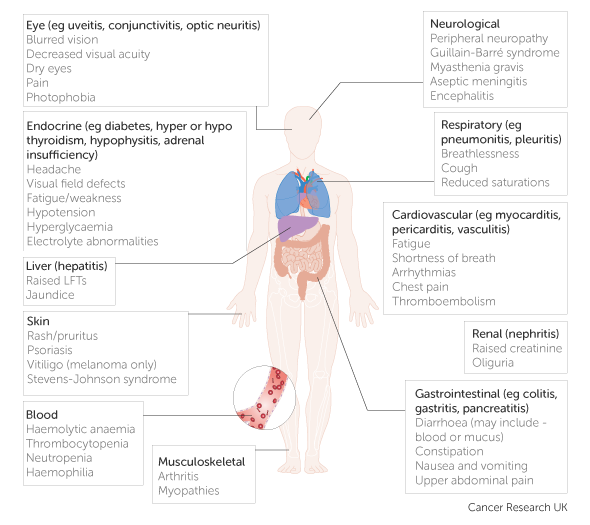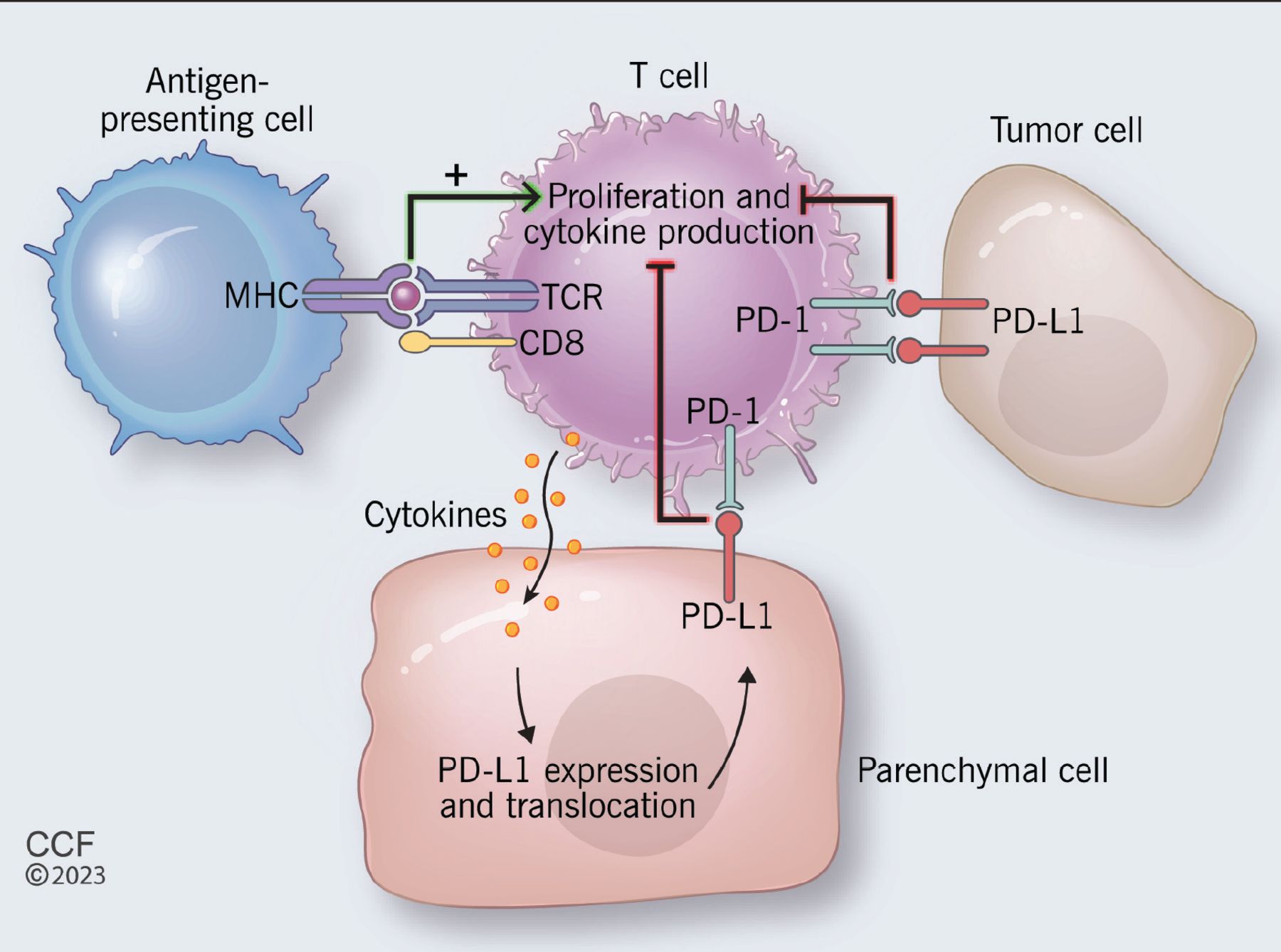
Endocrinopathies from checkpoint inhibitors: Incidence, outcomes, and management | Cleveland Clinic Journal of Medicine

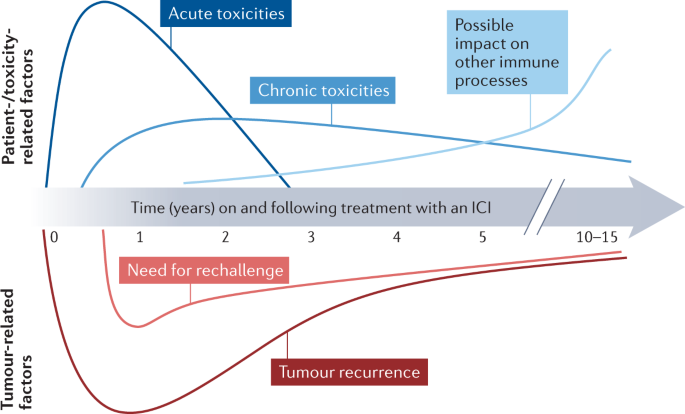
Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy - ScienceDirect
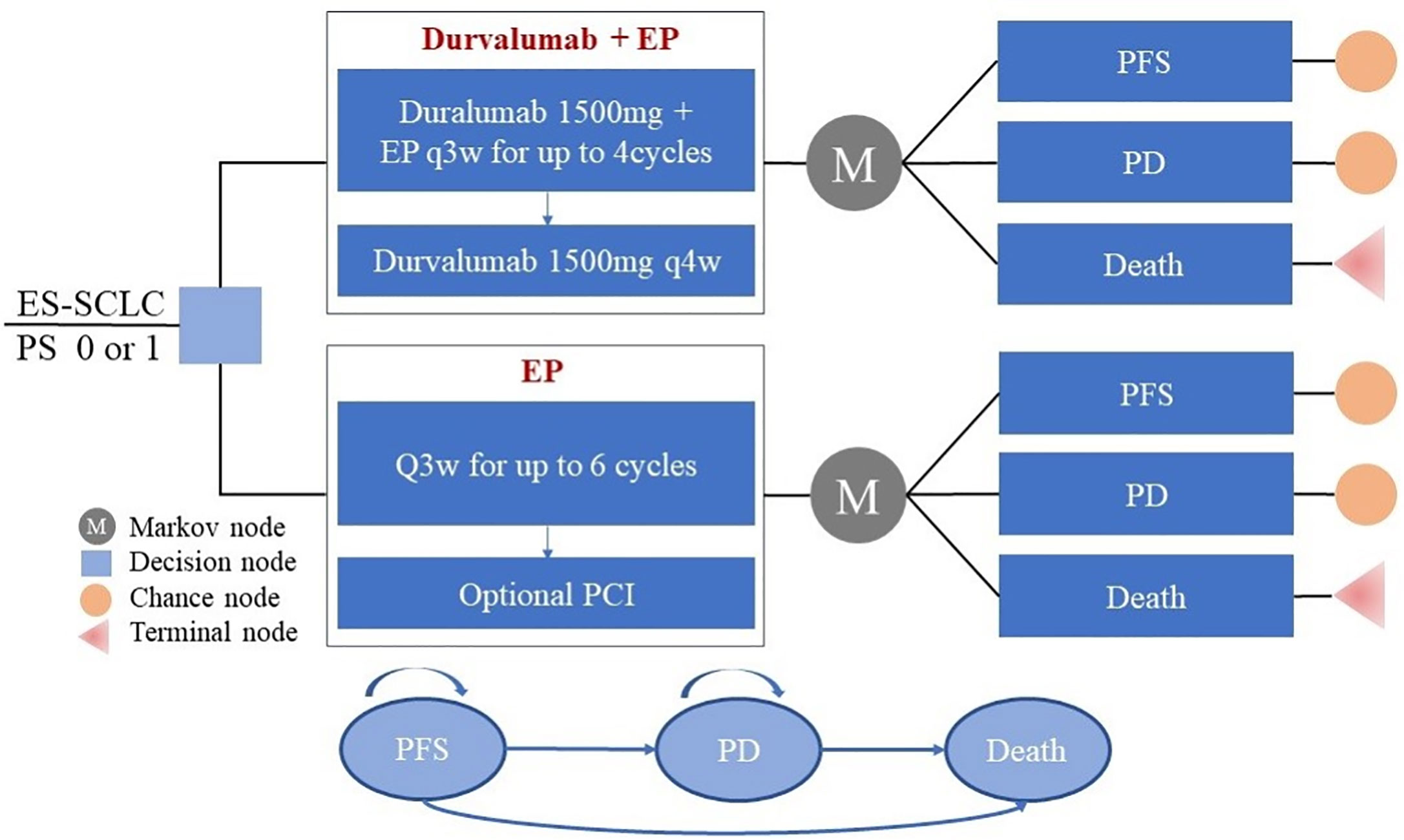
Frontiers | First-Line Durvalumab Plus Platinum-Etoposide Versus Platinum-Etoposide for Extensive-Stage Small-Cell Lung Cancer: A Cost-Effectiveness Analysis
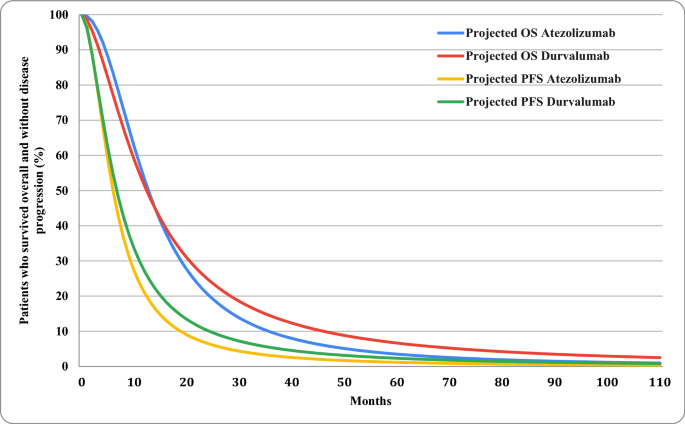
Cost-Effectiveness Analysis of Atezolizumab Versus Durvalumab as First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer in the USA | Clinical Drug Investigation
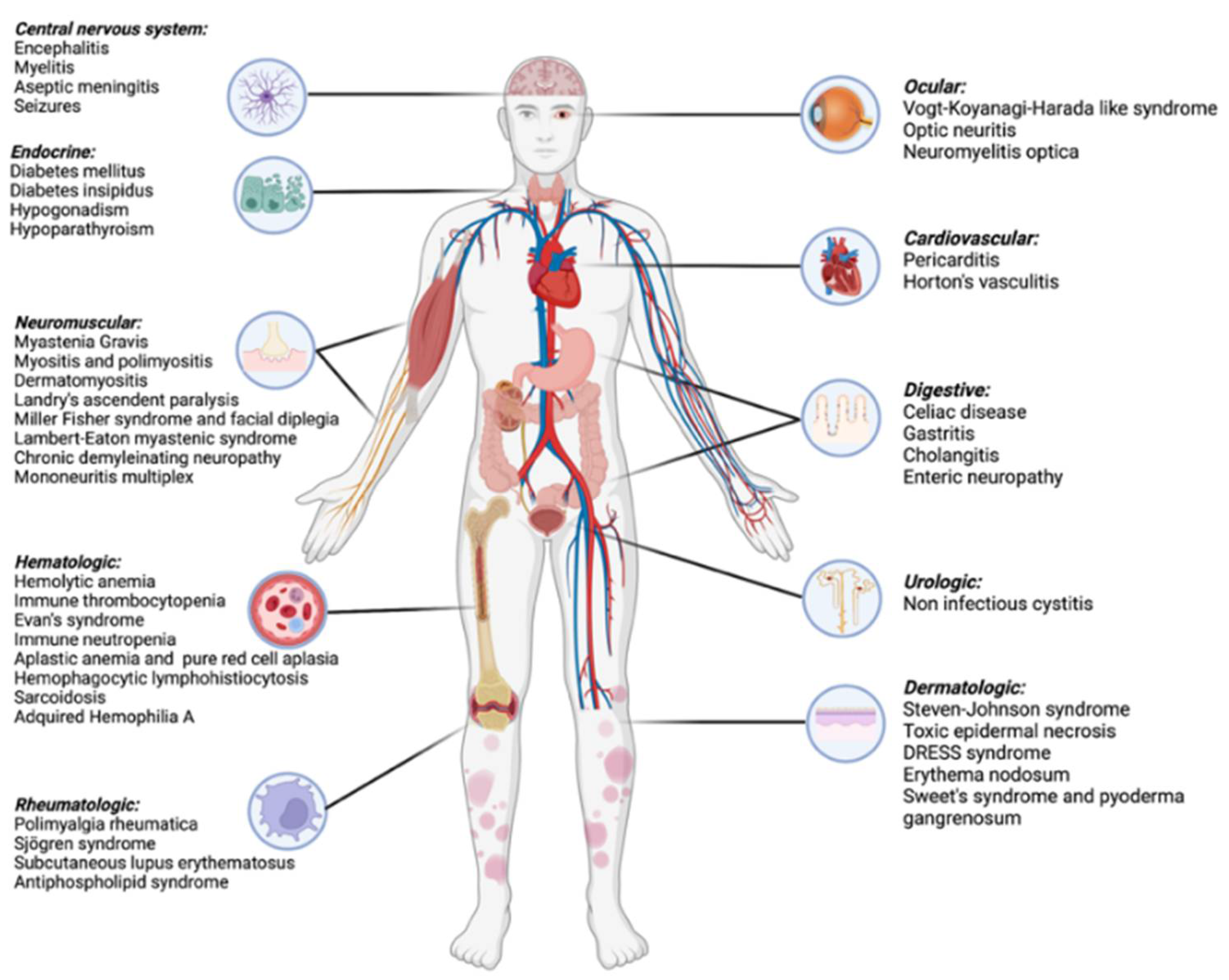
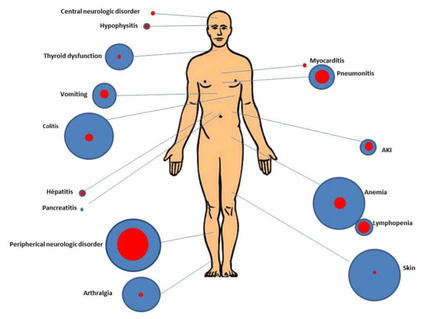
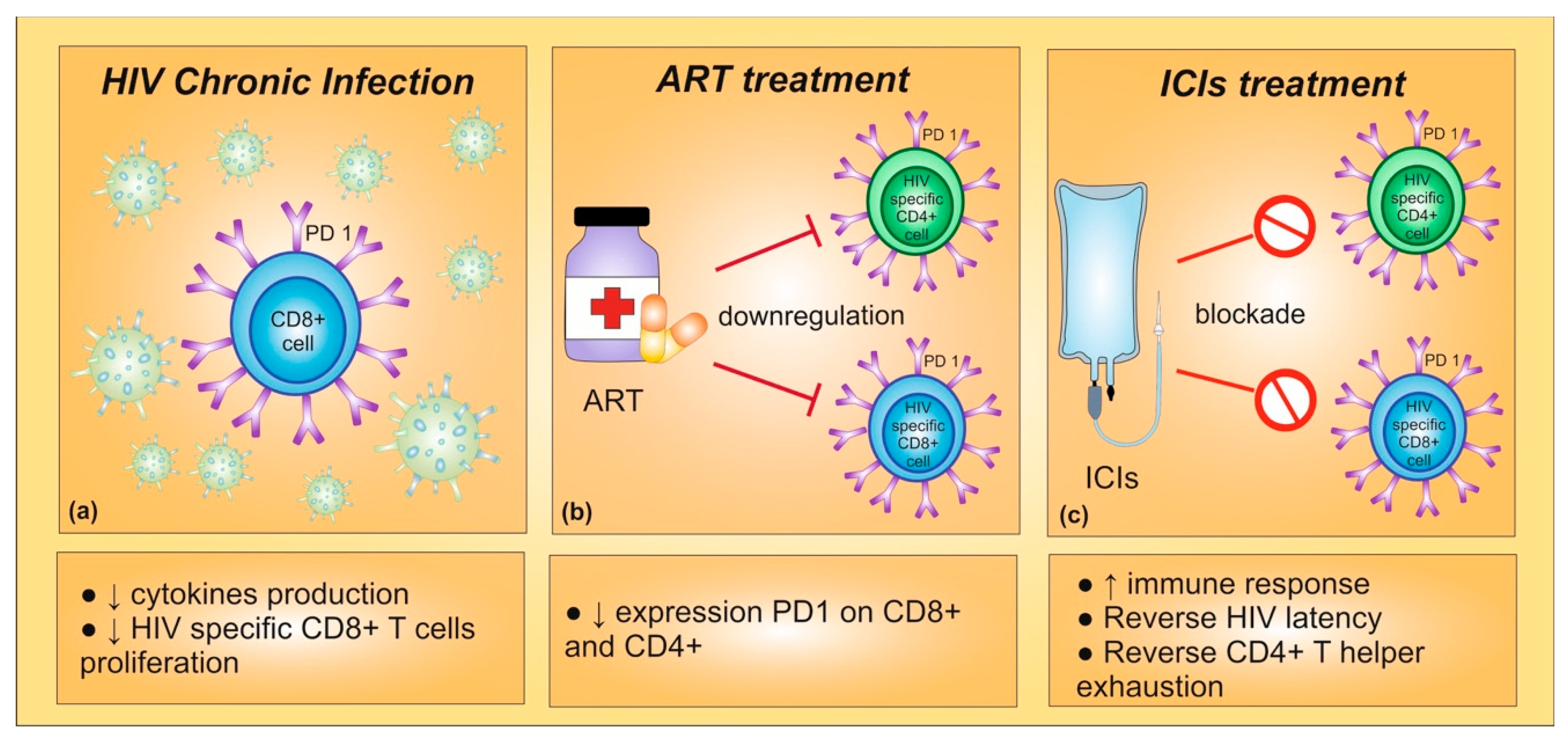
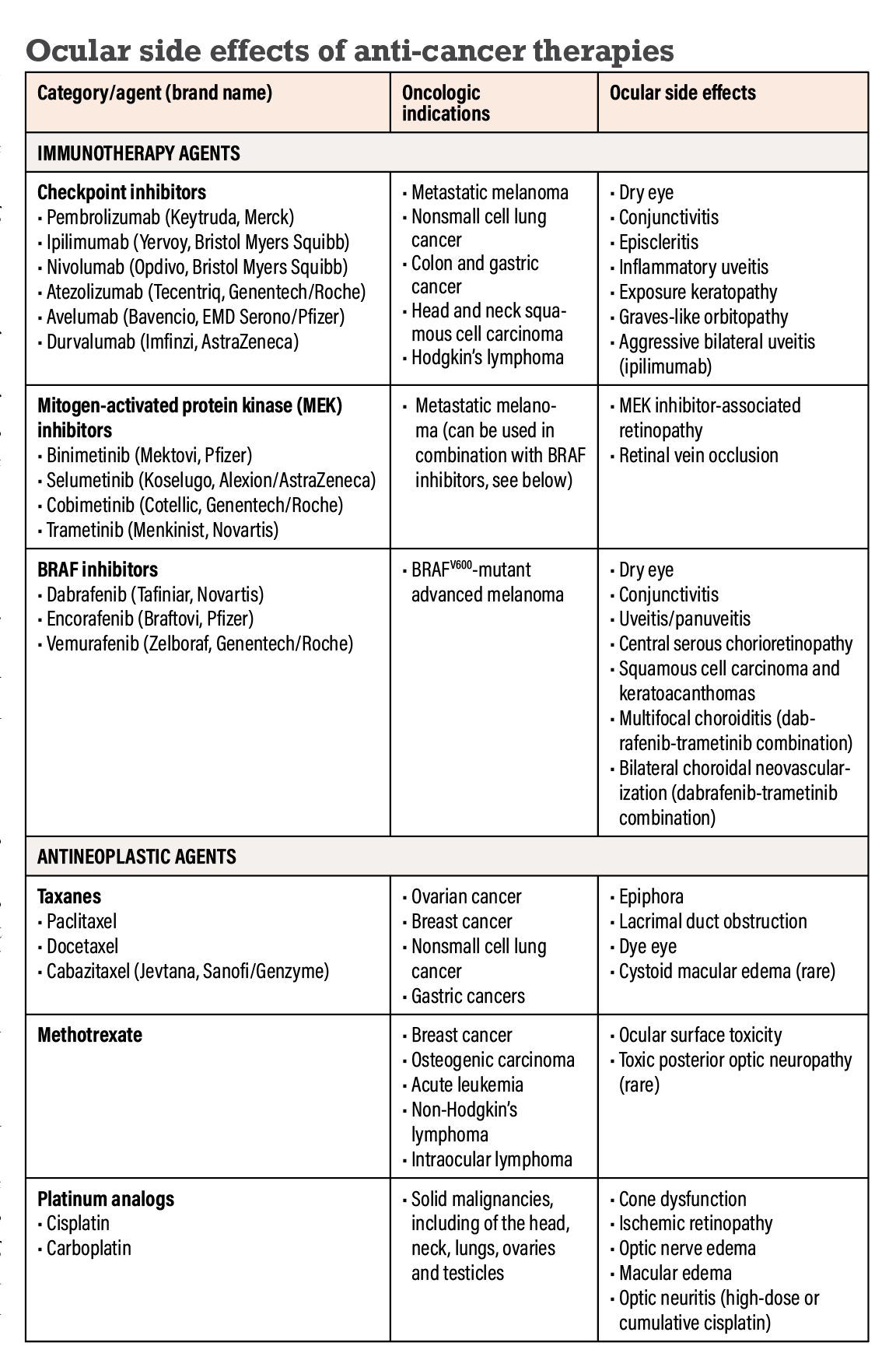
Diagnostics | Free Full-Text | Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy

Durvalumab Immunotherapy: Nursing Management of Immune-Related Adverse Events During the Journey of Patients With Stage III Non-Small Cell Lung Cancer | ONS

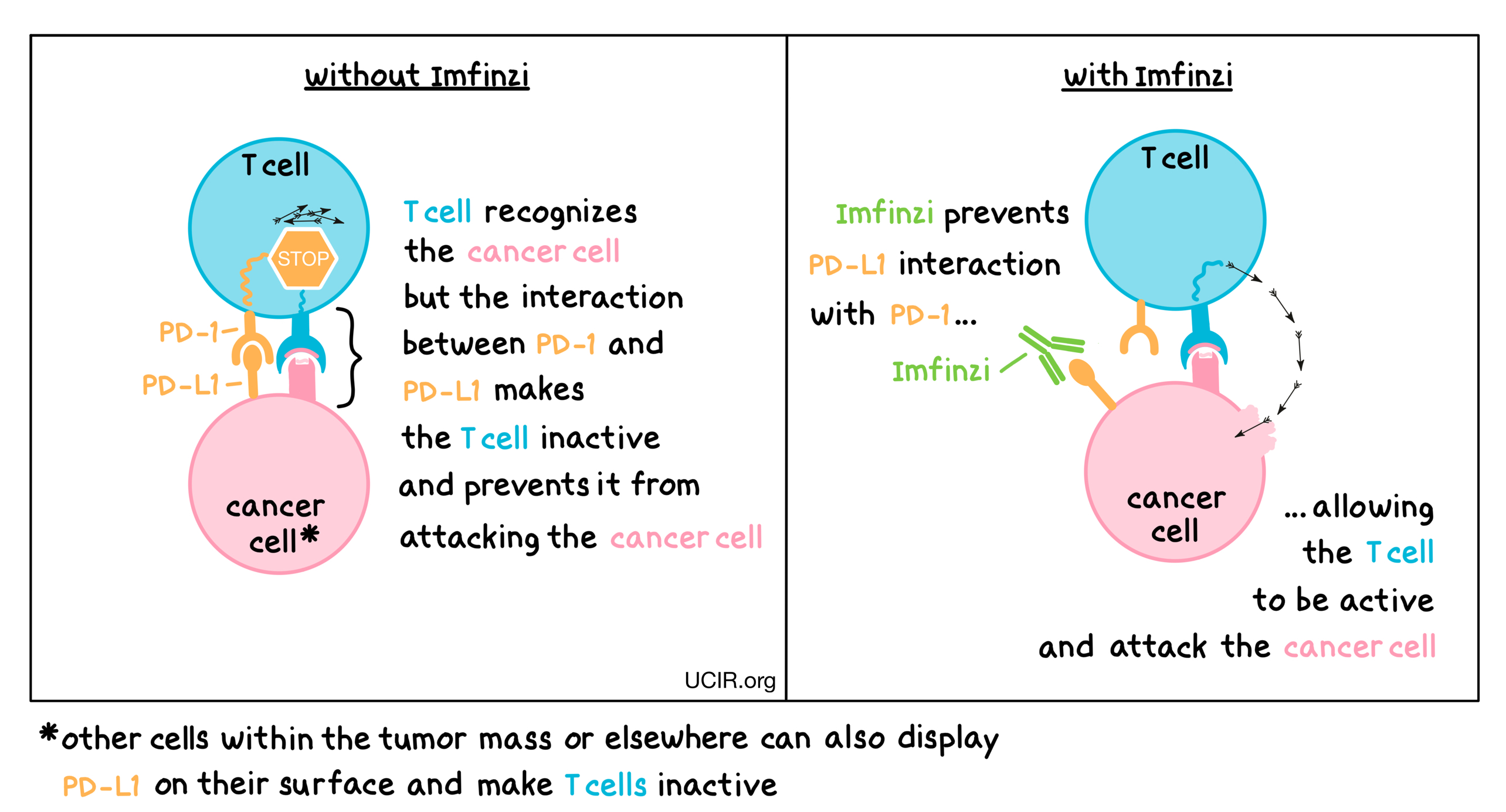
Clinical Activity, Tolerability, and Long-Term Follow-Up of Durvalumab in Patients With Advanced NSCLC - ScienceDirect

Reimagining Treatment for Unresectable Stage III Non-Small Cell Lung Cancer - Cancer Therapy Advisor

Patient-reported outcomes with durvalumab by PD-L1 expression and prior chemoradiotherapy-related variables in unresectable stage III non-small-cell lung cancer | Future Oncology

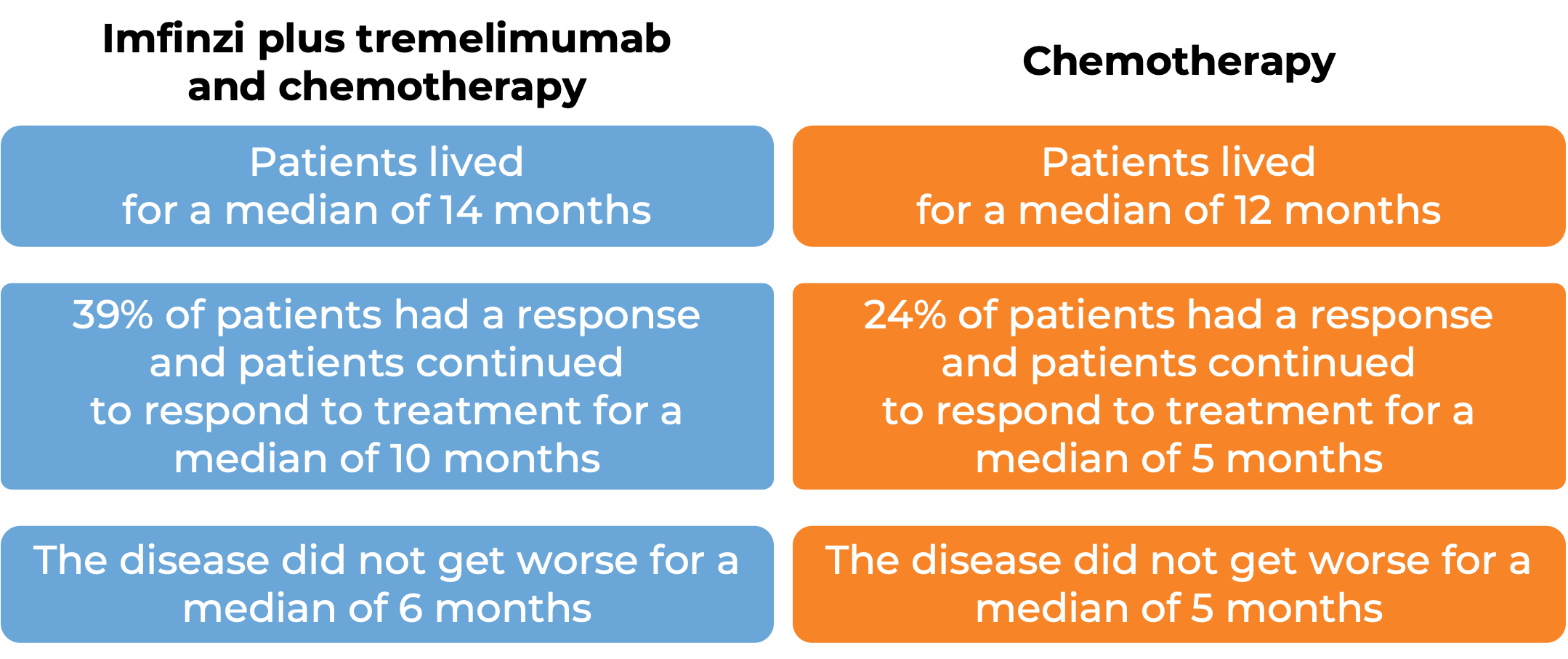
Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial ...